Articles from Thermedical

Thermedical®, a developer of advanced thermal-ablation technologies, today announced that the first patient has been treated in a pivotal clinical study evaluating its Saline Enhanced Radiofrequency (SERF) Ablation System with the Durablate® Catheter for ventricular tachycardia (VT) that continues despite medications and multiple prior ablation procedures. The procedure was performed at the Montreal Heart Institute by Katia Dyrda, MD, MSc, FRCPC, a cardiac electrophysiologist and associate professor at the Université de Montréal.
By Thermedical · Via Business Wire · December 16, 2025

Thermedical®, a developer of advanced thermal-ablation systems to treat ventricular arrhythmias, announced today that it has completed a feasibility study utilizing Pulsed Field Ablation (PFA) therapy in combination with its SERF Ablation System and Durablate® Catheter for treating ventricular tachycardia (VT). VT is an abnormally rapid heart rhythm that is a leading cause of sudden cardiac death worldwide.
By Thermedical · Via Business Wire · April 23, 2024

Thermedical®, a developer of thermal-ablation systems to treat ventricular arrhythmias, announced today it has received $3 million in grant funding (1R44HL169112) from the National Heart, Lung, and Blood Institute, a part of the National Institutes of Health, to fund its FDA-approved clinical trial at seven centers in the U.S. and Canada using the SERF Ablation System with the Durablate® Catheter to treat ventricular tachycardia (VT). VT is an abnormally rapid heart rhythm that is a leading cause of sudden cardiac death worldwide. Sudden cardiac death kills 325,000 adults in the U.S. every year1.
By Thermedical · Via Business Wire · October 10, 2023
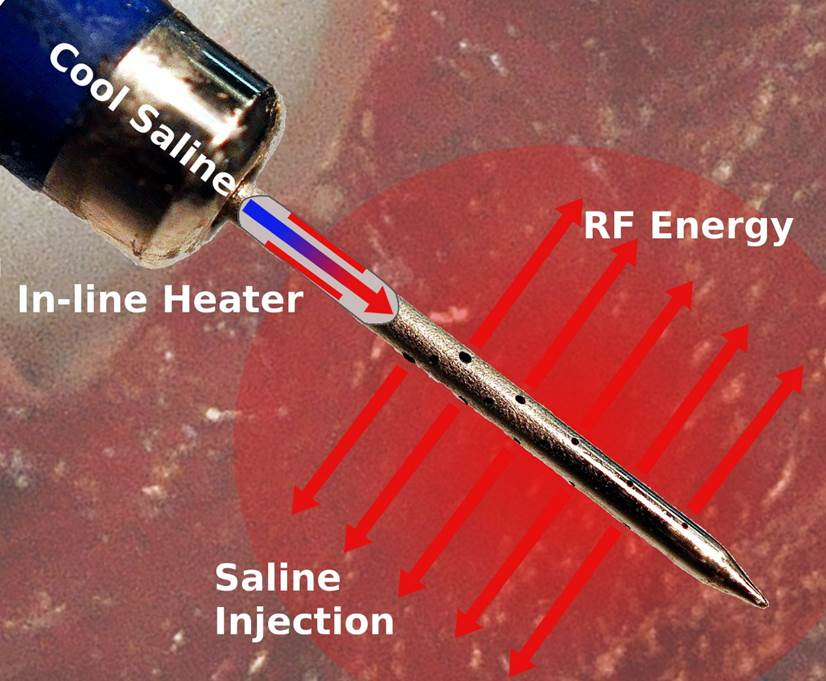
Thermedical®, a developer of thermal-ablation systems to treat ventricular arrhythmias, announced today that the U.S. Food & Drug Administration (FDA) has approved an open-label, single-arm interventional clinical trial to evaluate the safety and efficacy of the Thermedical® SERF Ablation System with the Durablate® Catheter in people with ventricular tachycardia (VT) resistant to conventional treatment. VT is an abnormally rapid heart rhythm that is a leading cause of sudden cardiac death worldwide.
By Thermedical · Via Business Wire · August 22, 2022
